ENCePP is the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance coordinated by the European Medicines Agency (EMA) aimed to strengthen the monitoring of the benefit-risk balance of medicinal products in Europe.


ENCePP is the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance coordinated by the European Medicines Agency (EMA) aimed to strengthen the monitoring of the benefit-risk balance of medicinal products in Europe.
On 31th May, 2017 the European Medicines Agency (EMA) published an action plan for small and medium-sized enterprises (SMEs), which aims to foster innovation and support SMEs in the development of novel human and veterinary medicines. This action plan builds on...
On 25th, July 2017, the European Medicines Agency (EMA) published a revision of the guidance on first-in-human clinical trials, that will come into effect on February 1st, 2018. The guidance addresses non-clinical issues for consideration prior to the first...
In July 2017, the Article “Tuberculosis treatment for children: An update” has been published on-line in “Anales de Pediatría” Journal. The publication reports the updated international guidelines for the management of paediatric Tuberculosis (TB) in Spain, reviewed...

[fusion_builder_container hundred_percent="no" equal_height_columns="no" hide_on_mobile="small-visibility,medium-visibility,large-visibility" background_position="center center" background_repeat="no-repeat" fade="no" background_parallax="none" parallax_speed="0.3"...
The article “Investigating the roles and training of paediatric research nurses working across Europe: a questionnaire-based survey”, aimed to identify potential training needs and compare the roles of research nurses across specialties and countries, has been...

Also this year the Gianni Benzi Pharmacological Research Foundation organises the annual Foresight Training Course (FTC), at its tenth edition now. The meeting, entitled ‘THE EUROPEAN MEDICINES REGULATORY NETWORK: PRESENT AND FUTURE’, will focus on: – the main...
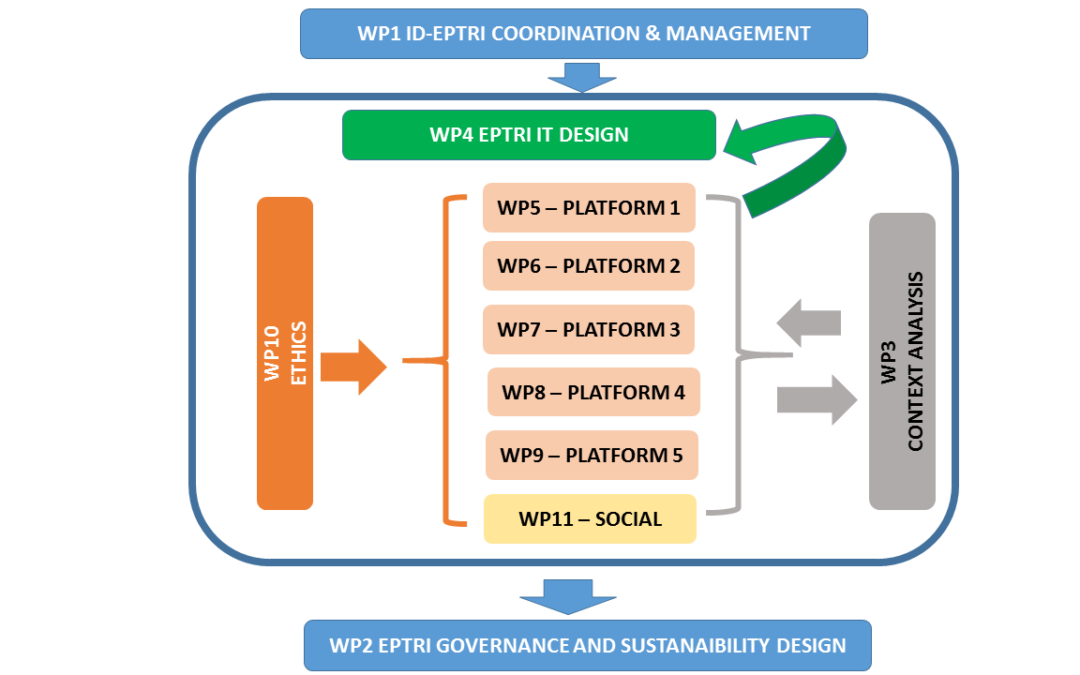
We are very pleased to announce that the ID-EPTRI (European Paediatric Translational Research Infrastructure) project, coordinated by CVBF and submitted within the INFRADEV-2016-2017 single-stage call for proposals, aimed to create the framework for a new paediatric...
The TEDDY network is working to launch a series of collaborative operating groups, focused on the most relevant topics of paediatric clinical research. This action is intended to convey the Network expertise and experience to deliver useful instruments for the...
From 1 June 2016 to 31 August 2016 the Directorate General for Health and Food Safety, DG SANTE launched a public consultation with the aim to seek the views of stakeholders and other interested parties, on the "Summary of Clinical Trial Results for Laypersons", which...